COVID19
Brazil rejects import of Russia’s Sputnik V vaccine citing ‘serious defects, inherent risks’
The Brazilian health regulator Anvisa has red-flagged the import of the Sputnik V, the Covid-19 vaccine developed by Russia. The health authority turned down the request made by state governors as Brazil is battered by a deadly second wave of the coronavirus virus. Anvisa’s five-strong board voted unanimously not to approve the Russian vaccine after technical staff had highlighted “inherent risks” and “serious” defects, citing a lack of information guaranteeing its safety, quality and effectiveness.
About 14 states in Brazil had appealed for an urgent import of Sputnik Vto counter a growing wave of COVID-19 infections. The country has registered 14.4 million confirmed cases of the virus and almost 400,000 deaths since the onset of the pandemic over a year ago.
Russia is using Sputnik V in its mass vaccination campaign, and the vaccine has been approved for emergency use in dozens of other countries including India. Notably, India drug regulator has approved the emergency use of Sputnik V. India is likely to receive the first batch of Russia’s Covid-19 vaccine ‘Sputnik V’ on May 1, Reuters reported citing officials of Russian Direct Investment Fund (RDIF).
The RDIF has said it expects production of the vaccine in India to reach 50 million doses a month by the summer and to rise further, Reuters reported. Its rollout has been embroiled in controversy, with President Vladimir V. Putin announcing its approval for use even before late-stage trials began. Russian scientists say it is 97.6% effective against Covid-19 in a “real-world” assessment based on data from 3.8 million people.
Also Read:
Russia to deliver Sputnik V COVID-19 Vaccine to India on 1 May
Reacting to the development, the official Sputnik V Twitter account retorted in a series of tweets in Portuguese, saying that the vaccine’s developers had shared “all the necessary information and documentation” with Anvisa. In another tweet, it said Anvisa’s decision “was of a political nature” and had “nothing to do with access to information or science.” It alleged that the United States had persuaded Brazil to deny approval.


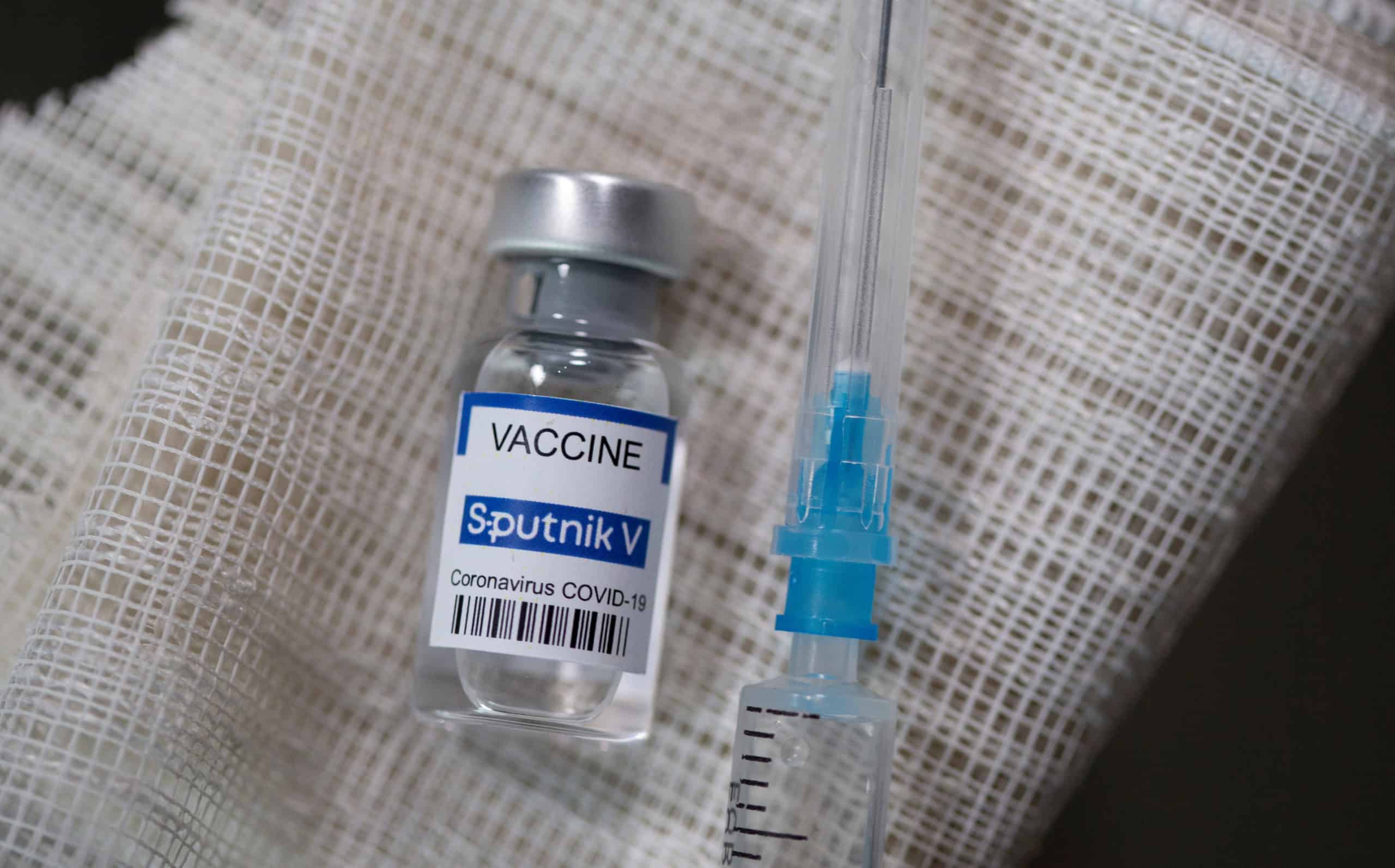









































Pingback: MSD to sign licensing pacts with 5 Indian drug firms for oral drug candidate for COVID-19 | The Plunge Daily