COVID19
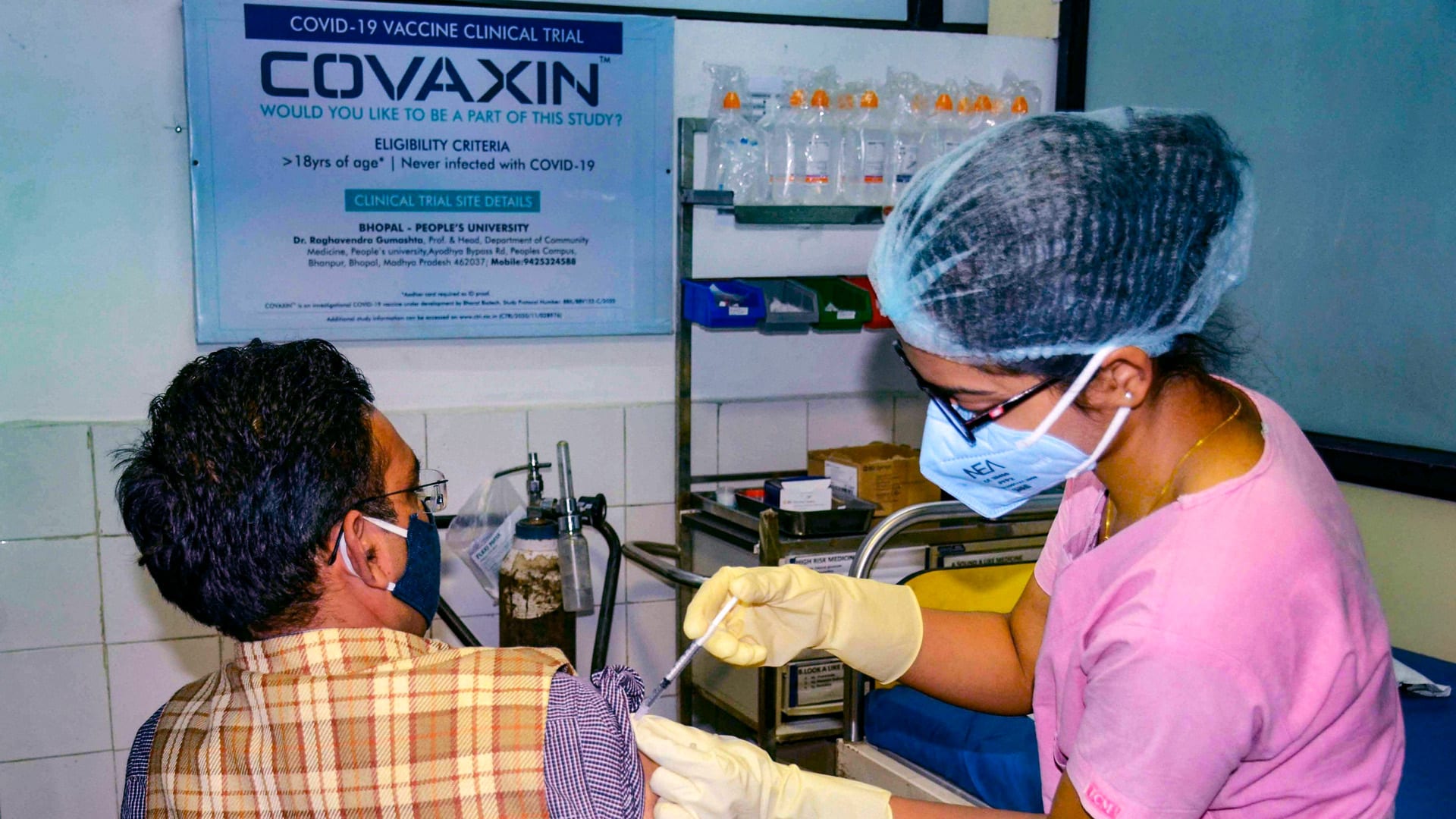
Covaxin to get WHO’s emergency use approval at earliest: Bharat Biotech
Bharat Biotech on Monday said it has submitted all documents required for the emergency use listing (EUL) of its COVID-19 vaccine Covaxin to the World Health Organization and expects a nod at the earliest. Last week, World Health Organization (WHO) chief scientist Soumya Swaminathan had said that the global health body is likely to take a decision on Bharat Biotech’s COVID-19 vaccine Covaxin in the emergency use listing within four to six weeks.
“All documents required for Emergency Use Listing (EUL) of Covaxin have been submitted to WHO as of 9th July. The review process has now commenced with the expectation that we will receive EUL from WHO at the earliest,” Bharat Biotech International Ltd Chairman and MD Krishna Ella said in a tweet posted by the company. According to WHO guidelines, EUL is a procedure to streamline the process by which new or unlicensed products can be used during public health emergencies.
Also read: Airtel clocks over 1Gbps speed during 5G trial in Mumbai
“We currently have six vaccines approved with EUL and have recommendations from our Strategic Advisory Group of Experts (SAGE). We continue to look at Covaxin. Bharat Biotech has now started uploading their data on our portal and that is the next vaccine that will be reviewed by our experts committee,” Swaminathan had said.










































Pingback: Oliveboard raises Rs 23 cr in funding round led by IAN Fund