COVID19
Coronavirus: India gives nod to 2 new vaccines and 1 anti-covid pill
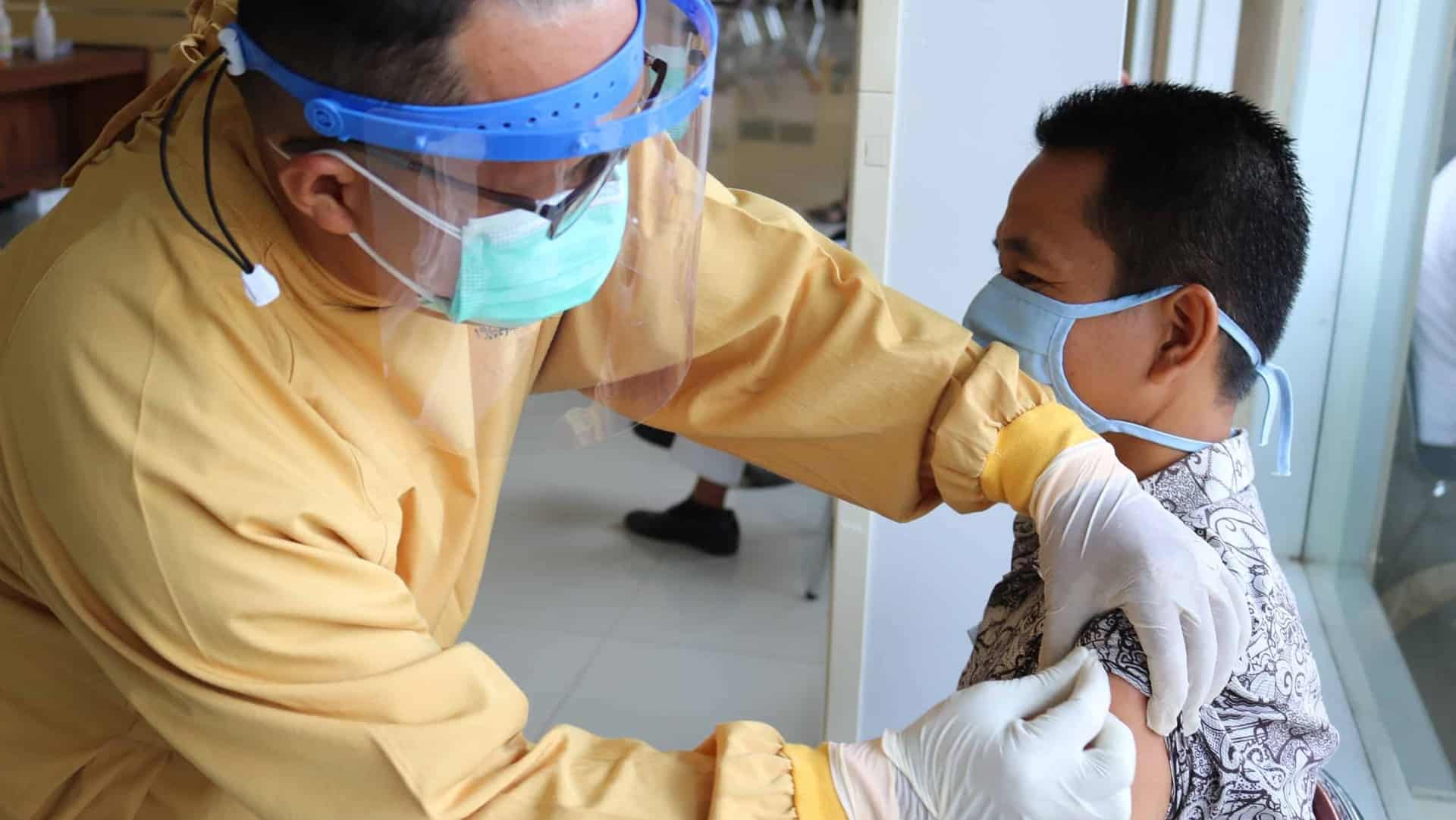
Union Health Minister Dr Mansukh Mandaviya on Tuesday announced that the Central Drugs Standard Control Organisation (CDSCO) had approved two more COVID-19 vaccines, Corbevax and Covovax, for restricted emergency use in India.
The CDSCO panel has granted emergency approval to the anti-COVID pill Molnupiravir for treatment of COVID patients in India.
Congratulations India 🇮🇳
Further strengthening the fight against COVID-19, CDSCO, @MoHFW_INDIA has given 3 approvals in a single day for:
– CORBEVAX vaccine
– COVOVAX vaccine
– Anti-viral drug MolnupiravirFor restricted use in emergency situation. (1/5)
— Dr Mansukh Mandaviya (@mansukhmandviya) December 28, 2021
“It’s a hat-trick! It’s now third vaccine developed in India,” Mr Mandaviya said. The other two vaccines developed in India are Bharat Biotech’s Covaxin and the Serum Institute of India’s (SII) Covishield.
Meanwhile, Molnupiravir will now be manufactured in the country by 13 companies for restricted use under emergency situation for treatment of adult patients with COVID-19 and who have high risk of progression of the disease, he informed.
All the recommendations have been sent to the Drugs Controller General of India (DCGI) for final approval, reported PTI.
Prakash Kumar Singh, director, government and regulatory affairs at SII, had submitted an application to the DCGI in October for grant of market authorisation for Covovax for restricted use in emergency situations. The World Health Organisation (WHO) had on December 17 issued emergency use listing for Covovax, expanding the basket of jabs validated by the global health body against the viral disease.
As for Biological E’s Corbevax, in light of the recommendations of the SEC meeting held on December 10, the firm submitted proposal for grant of marketing authorisation to the vaccine for restricted emergency use in adults along with the updated interim safety and immunogenicity data of phase 2/3 clinical trial and updated interim safety and immunogenicity data of phase 3 active comparator trial.
Also Read: Google moves Karnataka HC against CCI’s probe into Play Store rules
So far six COVID-19 vaccines — Serum Institute’s Covishield, Bharat Biotech’s Covaxin, Zydus Cadila’s ZyCoV-D, Russia’s Sputnik V and the US-made Moderna and Johnson and Johnson — have received emergency use authorisation from the Indian drug regulator.