Healthcare
Russian mRNA Cancer Vaccine Enteromix Sparks Hope — But Experts Urge Caution
Enteromix represents hope tempered with caution. The vaccine is a reminder that while science continues to push boundaries, breakthroughs in cancer treatment require patience, rigorous testing, and global cooperation.
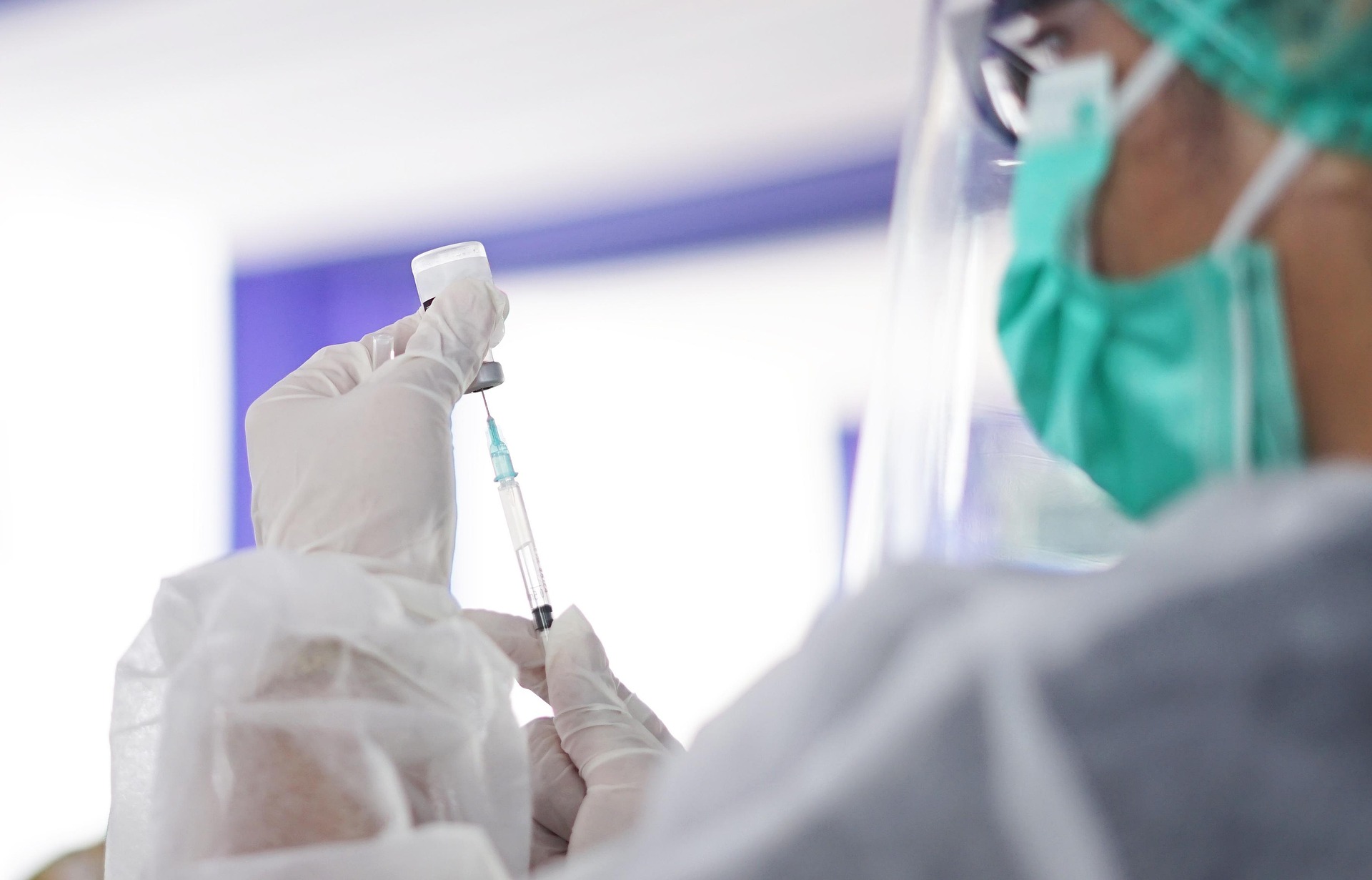
Russian researchers have unveiled what could be one of the most significant developments in oncology: an mRNA-based cancer vaccine named Enteromix. Early trial results suggest the vaccine has achieved 100% efficacy in shrinking tumors and slowing their growth, while being safe for repeated use. Initially developed for colorectal cancer, one of the world’s most common malignancies, Enteromix may soon expand into treating aggressive skin and brain cancers.
The announcement comes at a time when the global scientific community is divided on the future of mRNA technology, following the U.S. government’s recent decision, led by Robert F. Kennedy Jr., to restrict federal funding for such research. The Russian breakthrough, therefore, has sparked both optimism and scrutiny worldwide.
Why Experts Remain Skeptical
Despite the striking claims, oncologists and immunologists have urged caution. The cancer vaccine Enteromix trials were reportedly conducted on fewer than 50 volunteers, far too small a sample size to confirm universal efficacy. Cancer vaccines, particularly those tailored to individual immune systems, require extensive and diverse testing to validate safety and effectiveness.
Moreover, the results have not yet been published in a peer-reviewed journal, a crucial step before the scientific community can accept the findings as credible. Until that happens, experts argue, it would be premature to declare Enteromix a global breakthrough.
The Bigger Picture: mRNA in Cancer Research
The idea of using mRNA technology to fight cancer is not new. Scientists worldwide have been experimenting with mRNA vaccines for more than a decade, focusing on difficult-to-treat cancers like those of the pancreas, lungs, breast, and brain, as well as lymphomas.
The COVID-19 pandemic accelerated global interest in the technology, proving mRNA’s ability to enable rapid vaccine development. While traditional vaccines often take decades to perfect, mRNA platforms demonstrated that life-saving treatments could be manufactured and distributed within just two years.
Enteromix now joins a growing list of experimental cancer vaccines exploring how the immune system can be trained to recognise and destroy tumour cells.
🇷🇺 Russia’s Enteromix Cancer Vaccine Shows 100% Efficacy In Early Trials.
Enteromix is designed to train the immune system to recognize and eliminate cancer cells, a safer, more intelligent alternative to traditional treatments like chemotherapy.
The vaccines are initially…
— World of Statistics (@stats_feed) September 9, 2025
What Comes Next
If larger-scale trials confirm Enteromix’s reported success, it could revolutionize cancer treatment, shifting the narrative from expensive, invasive therapies to personalized vaccines tailored for each patient. However, the road ahead is long: regulatory approvals, global peer review, and international collaboration will be critical in determining whether this Russian venture lives up to its promise.
For now, Enteromix represents hope tempered with caution. The vaccine is a reminder that while science continues to push boundaries, breakthroughs in cancer treatment require patience, rigorous testing, and global cooperation.































































Pingback: New Drug Trio Show Promising Results Against Pancreatic Cancer