Business
Global Pharma recalls 50,000 tubes of eye ointment in US: USFDA
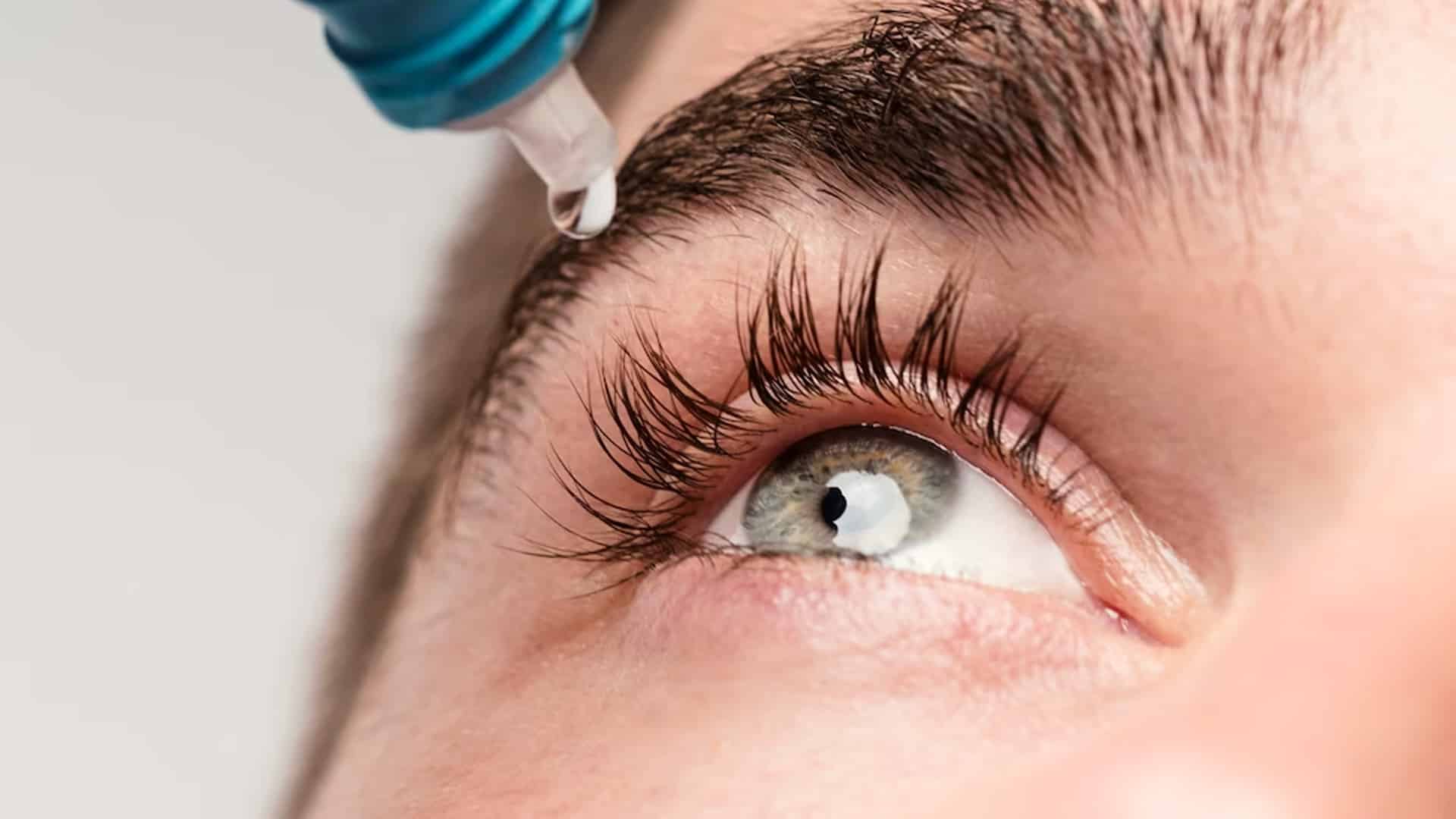
Global Pharma Healthcare is recalling 50,000 tubes of eye ointment in the US market due to bacterial contamination, according to the US Food and Drug Administration (USFDA).
As per its latest Enforcement Report, the US health regulator noted that the Chennai-based drug firm is recalling the affected lot of “Artificial Eye Ointment” in the US market. The lot has been manufactured by Chennai-based Global Pharma Healthcare and distributed in the US market by New York-based Delsam Pharma, the USFDA said. Stating the reason for recall, the US health regulator said: “FDA analysis found unopened tubes to be contaminated with bacteria.”
Also read: Smartworks plans to raise USD 70-90 mn this fiscal for growth; FY’23 revenue rises to cross Rs 700cr
The company initiated the Class I recall on February 24 this year. As per the USFDA, a Class I recall is the most urgent of the three types of FDA recalls and usually pertains to defective products that can cause serious health problems. In a separate disclosure, the USFDA stated that Mumbai-based Sun Pharma is recalling 1,920 bottles of Dofetilide Capsules, which are used to treat irregular heartbeat.
The affected lot has been produced at Sun’s Dadra-based plant, the USFDA stated. The US-based unit of the company — Sun Pharmaceutical Industries Inc — is recalling the lot due to “Failed content uniformity specifications,” it added. The New Jersey-based firm initiated the Class III recall on March 9. As per USFDA, a Class III recall is initiated in a “situation in which use of, or exposure to, a violative product is not likely to cause adverse health consequences.”