COVID19
Zydus Cadilla’ Virafin gets emergency use approval for treating moderate Covid cases
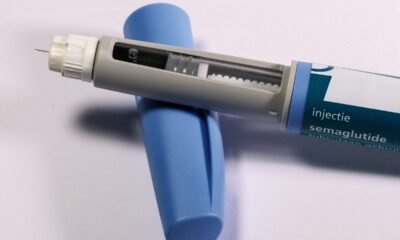
Amid an unprecedented surge in covid infections, Indian pharma major Zydus Cadila has announced that it has received restricted emergency use approval from the Drug Controller General of India (DCGI) to use the antiviral drug Virafin for the treatment of moderate COVID-19 infections.
A single-dose subcutaneous regimen of the antiviral Virafin will make the treatment more convenient for the patients, Zydus Cadila said in a regulatory filing. When administered early on during COVID-19, Virafin will help patients recover faster and avoid much of the complications, the company said.
Virafin drug was also found to reduce hours of supplemental oxygen required by patients. The drug, when given to Covid patients in the early stage, has shown significant clinical and virological improvement in moderate cases. According to the drug maker, patients who were treated with Virafin were tested negative within 7 days.
Speaking on the development, Dr. Sharvil Patel, Managing Director, Cadila Healthcare Limited said “The fact that we are able to offer a therapy which significantly reduces viral load when given early on can help in better disease management. It comes at a much-needed time for patients and we will continue to provide them access to critical therapies in this battle against COVID-19.”
In its Phase III clinical trials, the therapy had shown better clinical improvement in the patients suffering from COVID-19. During the trials, a higher proportion of patients administered with PegIFN arm were RT PCR negative by day 7. The drug ensures faster viral clearance and has several add-on advantages compared to other anti-viral agents.
Also Read:
DCGI gives nod to Zydus Cadila for phase 3 clinical trials with biological therapy for COVID-19 vaccine
The pharma company said that the Pegylated Interferon alpha-2b (PegIFN) Virafin will be available only on the prescription of medical specialists and is meant for use in institutional setups or hospitals. PegIFN has very well-established safety with multiple doses in chronic Hepatitis B and C patients for many years.